Development Overview - Your Ideas Consulting
Main menu
- Home
-
Product Development
- Development Overview
- by Phase Results:
- Proposal
- Feasibility
- Realization
- Validation
- Commercialization
-
Process Development
- Processes Overview
- by System Processes
- Requirements
- Product Risks
- Verification & Validation
- Product Configurations
- Consulting
- Training
-
About
- Mission
- Management
- Juergen Friedrichs
- Advisory Board
- Knut Bartl
- Alfred Lang
- Guenther Schatz
- Copyright
- Contact
Development Overview
Product Development Overview
We support your IVD product development with the provision of results/ document deliverables during the full lifecycle of the product. This service potentially targets:
new developments,
existing products,
also products, moving from research level to an IVD labeled product.
Our major focus is linked to the provision of key deliverables/ documents , which provide structure and interdisciplinary integration for the project as well as for the product.
The deliverables are mainly linked to the four processes of IVD system development:
Further focus is directed to the analysis and interdisciplinary confirmation of requirements linked to high level design contents in early project phases e.g. compilation of the results/ deliverables:
reliability analysis,
product scenario alternatives,
throughput analysis,
safety design,
automation roadmap,
especially considering solution alternatives and customer needs.
Deliverables of one process are matched regularly with plans and reports of the other three processes.
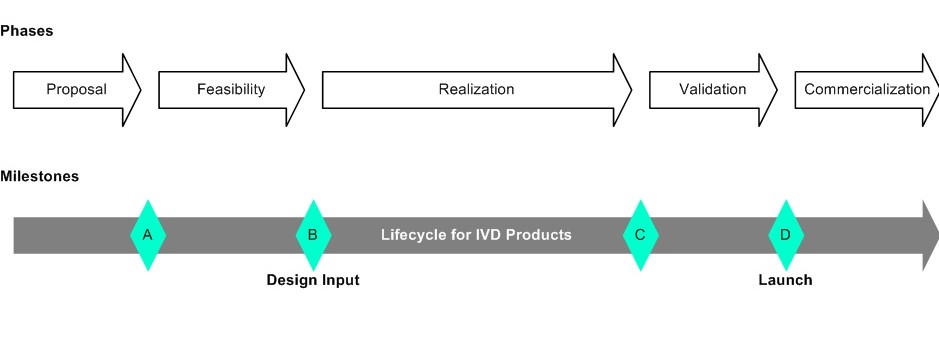
A simple design control model is provided below to later on sort deliverables with their initial creation accordingly into phases.
Design Control Model
A simple design control model was defined with the following phases and milestones:
Proposal
During this mainly business driven phase market analysis leads to different product scenarios with related business cases and high level concepts and first customer-
and product requirements. Successful end of phase is followed by the beginning of a feasibility phase, approved by senior management.
Feasibility
Existing content (e.g. business case, market analysis, requirements, concepts) is further refined and pre-
product components are provided and utilized to proof initial product performance. These activities now require substantial R&D effort; an interdisciplinary team is set up including serious project management resources.
Alternative scenarios/ realizations are still considered, with the goal to present the best product solution at end of phase.
Requirements are defined on customer and product level down to component requirements (hardware, software, reagents, disposables) as design input artifacts on an approx. 90% level, feasibility reports document accomplished performance.
A product risk analysis is provided on 90% level.
Successful end of phase is followed by the beginning of a formal realization phase.
Realization
Formal development is introduced with this phase by creation of compliant design control documentation under version control and also applied version control for all product components (hardware, software, reagents, disposables).
Project management is challenged to coordinate R&D resources and manufacturing interfaces.
Work breakdown of development activities and V&V activities is critical.
Requirement changes still do occur. Verification of individual components (hardware, software, reagents, disposables) is part of this phase and executed as planned.
Substantial R&D effort is consumed by end of phase.
Successful end of phase is followed by the beginning of a validation phase.
Validation
Final components (developed & manufactured) will be integrated into a product as planned.
Validation activities are executed and reported as planned (design output artifacts) .
Late changes challenge the whole team and venture.
Design control documentation needs to be finalized including successful passing of all V&V activities also considering deviations.
Usability is shown, clinical utility is shown.
Preparation of market introduction by interfacing with vendors, customers and approval authorities, also executing planned stock up of materials and components is critical for launch.
Successful end of phase is followed by the product launch.
Commercialization
Product is manufactured.
Product is sold to customers.
Lifecycle management proceeds still further executing change management within the four development core processes listed above.
Deliverable Overview
Following table lists project critical deliverables:
# |
Deliverable |
Phase |
Description |
1 |
Automation Strategy |
Proposal |
Analysis, plan and rationale for the provision of automation elements (e.g. instrumentation, hardware, software, disposables, customer workflow) utilized in existing and/or new products in a timely order. May be linked with assay portfolio plan. |
2 |
Product Scenario |
Proposal |
Analysis of solution space for a product in scope. Identify and decribe product alternatives by applying different target focus e.g. time to market. Refine individual scenarios with e.g. features, automation, R&D effort prior to further facilitate selection. |
3 |
Customer Workflow Analysis |
Feasibility |
Functional analysis and compilation of manual and automated steps for the customer application, performed by operators in lab environment |
4 |
Technology Assessment |
Proposal |
Comprehensive technical assessment/ compilation of a technology and/ or prototype IVD instrumentation system ( e.g magnetic bead capturing, detection) with: |
5 |
Intellectual Property Analysis |
Proposal |
Analysis of selected intellectual property content regarding: |
6 |
Requirements (Product and/ or Components) |
Proposal |
Requirements documents as defined in the requirements management plan on e.g. customer- |
7 |
System Design Scenarios |
Feasibility |
Analysis of solution space for automation architecture. Identify alternatives by applying different target focus e.g. single tube processor, throughput variants, batch analyzer, modularization, disposable variants. Refine individual scenarios with e.g. features, automation, R&D effort prior to further facilitate selection decision |
8 |
Safety Design Scenarios |
Feasibility |
Analysis and compilation of failure detection (single failures) methods and concepts to be applied. |
9 |
Product Risk Analysis |
Feasibility |
Compilation of product risk analysis according to ISO 14971 containing risks estimates and mitigation measures. |
10 |
Event Risk Management |
Realization |
Event driven risk analysis for individual (design) changes occurring from end of realization phase. Assessment links and tracks into change control for requirements, V&V documents, version control of components and respective revalidation effort, confirms successful mitigation of change. |
12 |
Requirements Management Plan |
Feasibility |
Defines requirements management process for a specific projects, including: |
13 |
Requirements Management Report |
Feasibility |
Reports execution of requirements management process for milestones. |
14 |
Risk Management Plan |
Feasibility |
Defines plan for execution of product risk management for a specific project: |
15 |
Risk Management Report |
Realization |
Reports execution of product risk management process for milestones. |
16 |
Master Validation Plan |
Feasibility |
Breakdown of all V&V activities for a product (platform & assays) with its components including manufacturing. Document may link into sub plans. |
17 |
Master Validation Summary Report |
Realization |
Bottom up reporting of all V&V activities executed, with pass/ fail indicators provided and deviations described. Document may utilize sub reports for reporting. |
18 |
Validation Plan (Component) |
Realization |
Sub plan of V&V activities for a component. Document may link into sub plans or protocols. |
19 |
Validation Plan Summary Report (Component) |
Realization |
Sub report for execution of V&V activities for a component. Document may utilize sub reports for reporting. |
20 |
Configurations Management Plan |
Feasibility |
Plan for product configuration scope and release along the lifecycle. Describes interfacing of platform versions with reagent lots. Plans introduction of upcoming changes (design change, obsolescence ...) in configurations. |
21 |
Configurations Management Report |
Feasibility |
Reports execution of configurations management process for milestones. |
22 |
Reliability Analysis |
Feasibility |
Analyses and defines reliability factors throughout the product (platform & assay), assigns variation estimates, reports overall expected variation. |
23 |
Project Plan |
Proposal |
Plan for project (assay and platform); including team organization, resources, applied design control and processes, vendors, budget plan, schedules, milestones. |