Feasibility - Your Ideas Consulting
Main menu
- Home
-
Product Development
- Development Overview
- by Phase Results:
- Proposal
- Feasibility
- Realization
- Validation
- Commercialization
-
Process Development
- Processes Overview
- by System Processes
- Requirements
- Product Risks
- Verification & Validation
- Product Configurations
- Consulting
- Training
-
About
- Mission
- Management
- Juergen Friedrichs
- Advisory Board
- Knut Bartl
- Alfred Lang
- Guenther Schatz
- Copyright
- Contact
Feasibility
Phase Feasibility
Compilation and consideration of the following deliverables is recommended during this phase:
Requirements need to be compiled comprehensively on a product/ system level e.g. by using a content template.
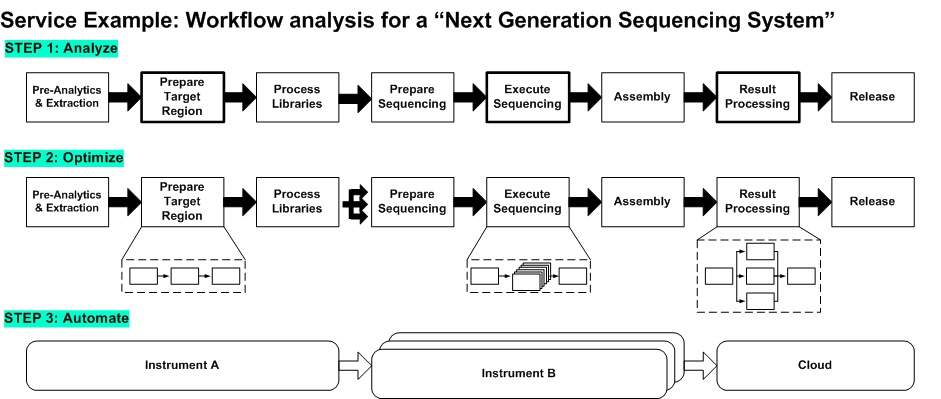
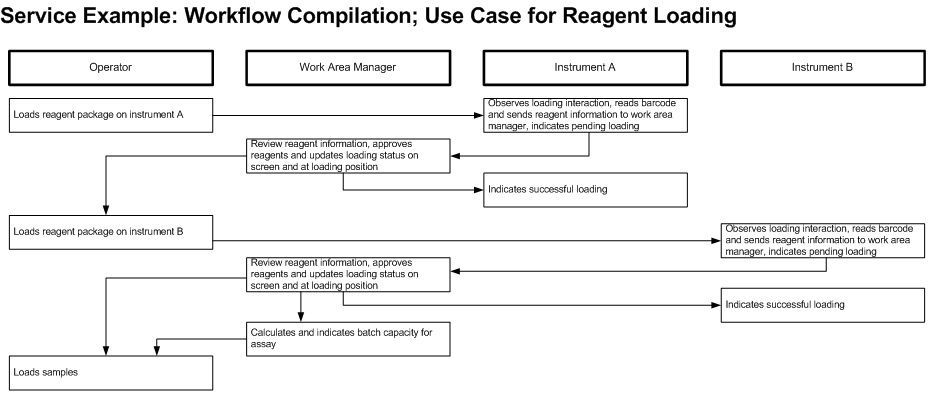
Critical contents to be considered:
-
-
-
-
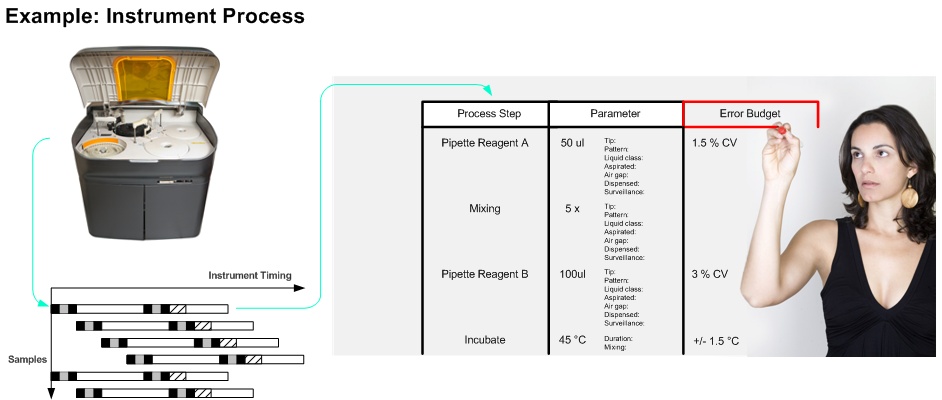
(e.g. reagent onboard time variation, pipetting variation, sample stability)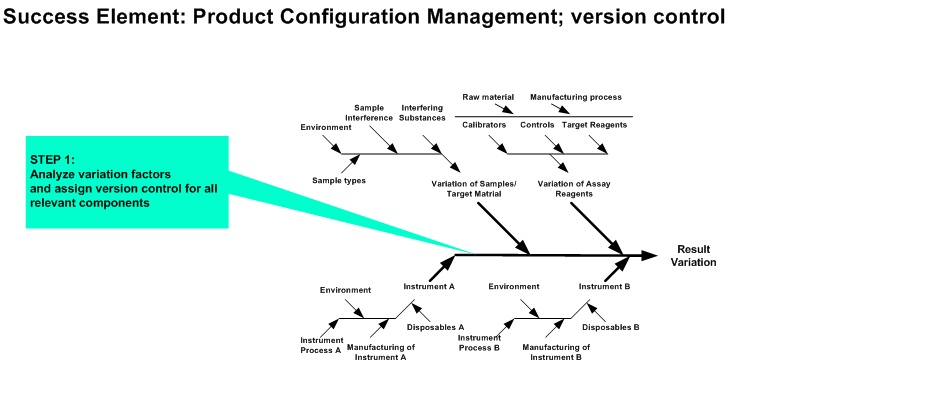
-
-
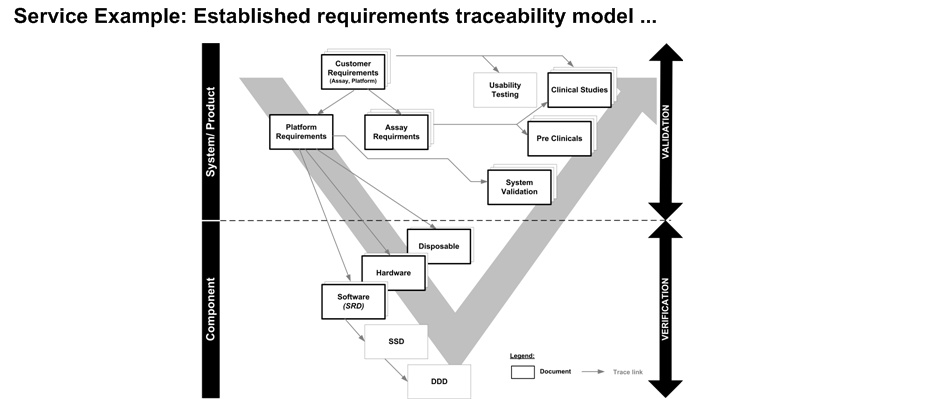
The requirements management plan defines the requirements process for a specific projects, including:
-
-
-
-
-
-
-
The Master Validation Plan provides a breakdown into all V&V activities (see layer model below) for a product (platform & assays) with its components, including manufacturing. 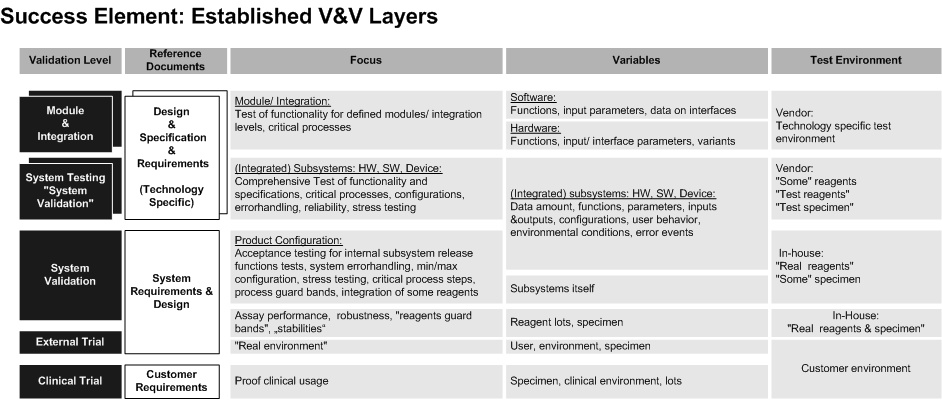
This planning process will result in a hierarchy of V&V plans (see below).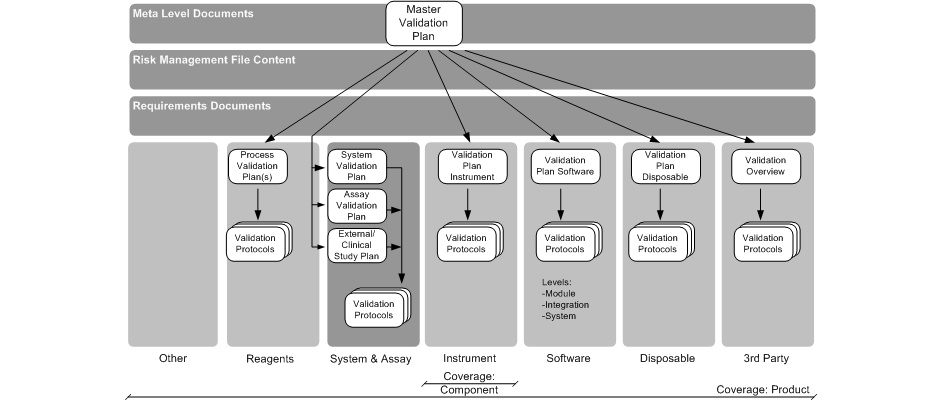
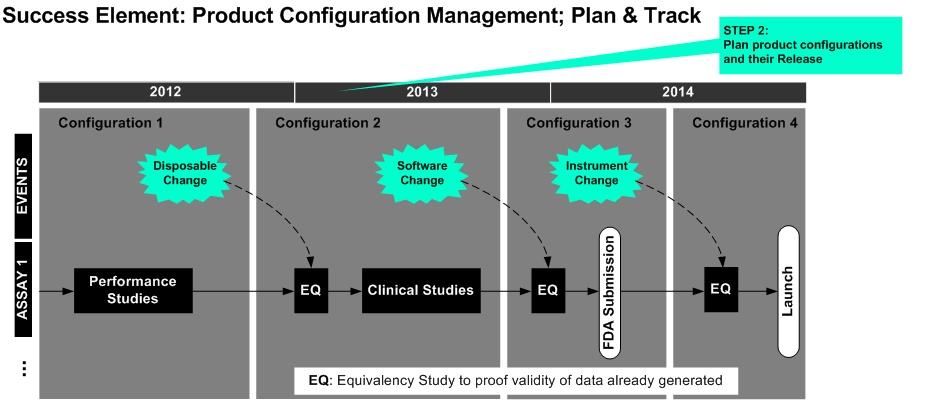
The Configuration Management Plan contains definitions and descriptions of product configuration scope and release along the lifecycle.
The interfacing of platform versions with reagent lots is provided . The document plans the introduction of upcoming changes (design changes, obsolescence ...) in configurations.