Processes Overview - Your Ideas Consulting
Main menu
- Home
-
Product Development
- Development Overview
- by Phase Results:
- Proposal
- Feasibility
- Realization
- Validation
- Commercialization
-
Process Development
- Processes Overview
- by System Processes
- Requirements
- Product Risks
- Verification & Validation
- Product Configurations
- Consulting
- Training
-
About
- Mission
- Management
- Juergen Friedrichs
- Advisory Board
- Knut Bartl
- Alfred Lang
- Guenther Schatz
- Copyright
- Contact
Processes Overview
Process Development Overview
We support your Medical Device/ IVD product development processes with the following services:
Process analysis and proposal for improvements,
Responsible execution of the process within your project/ team: Set up, plan, report and tweak the process with its deliverables,
and Training
for the system development processes:
Requirements Management,
Product Risk Management,
Verification and Validation,
Product Configuration Management.
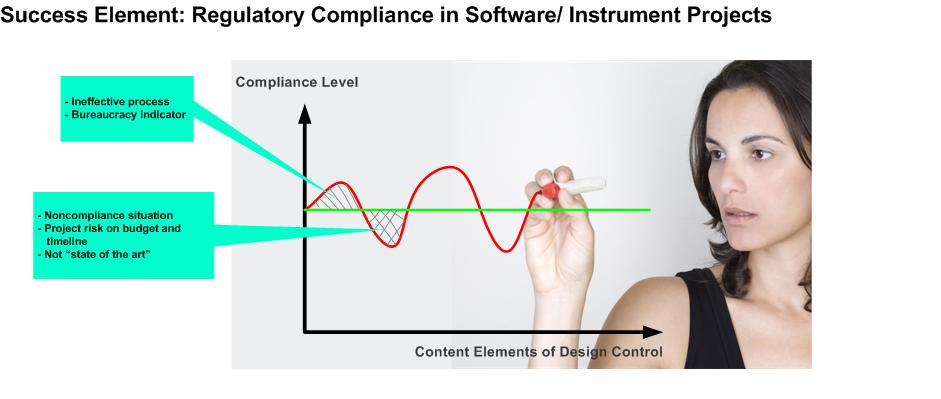
We apply a strong interdisciplinary perspective (hardware, software, reagents, disposables), which is accompanied by a mandatory adjustment:
to the complexity level of your product,
the linked regulatory strategy for your product,
as well as to specific needs of your organization/ departments.
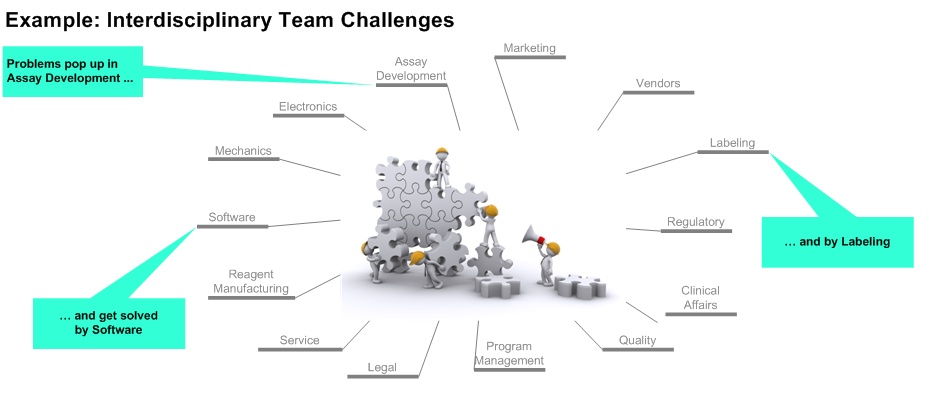
Our activities and deliverables provided for one process will be matched with content elements, organization and documentation of the other there processes, e.g.:
Requirements Management Plan matching overall traceability including risk mitigation measures,
V&V document structure matching requirement documents levels,
Product configuration planned matching requirements changes, event driven risk assessment and respective re-
validation effort, plan and reports.
It is critical to scale the process execution for an individual venture according to the required compliance level.